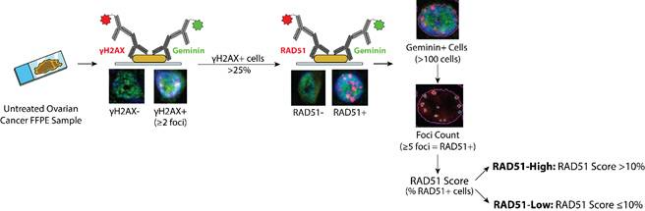
- Current biomarkers to predict response to standard-of-care platinum chemotherapy in high-grade serous ovarian cancer have limited accuracy. Our lab has worked with collaborators to develop a functional homologous recombination deficiency assay using RAD51 foci. Our preliminary work demonstrates that this reliable and reproducible RAD51 foci assay can predict platinum chemotherapy response in established and patient-derived ovarian cancer cell lines, patient-derived organoids, and pre-treatment formalin-fixed, paraffin-embedded tumor biopsies. Further, we developed a novel automated system to quantify this assay to facilitate translation into clinical care. We are now working to translate these findings into clinical care via prospective clinical trials. Additionally, we are working to identify additional markers to refine the accuracy of this biomarker.
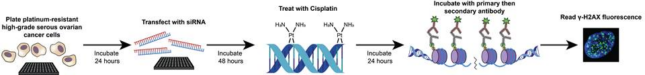
2. Targeting deubiquitinating enzymes and kinases to overcome platinum chemotherapy resistance in ovarian cancer – We performed a high-throughput screen in chemotherapy-resistant high-grade serous ovarian cancer cells. We used short-interfering RNA to knock down 216 DUBs and 720 kinases and then treated the cells with cisplatin and stained for γH2AX, a marker of DNA damage. We identified 7 DUBs and 89 kinases whose knockdown resulted in increased platinum-induced DNA damage. We have refined this list using patient-derived ovarian cancer samples to determine the most clinically relevant. We are currently working in the lab to validate these targets and create novel inhibitors for use in clinical care.
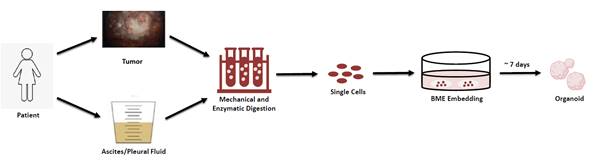
3. Elucidating mechanisms of poly (ADP-ribose) polymerase (PARP) inhibitor-induced platinum chemotherapy resistance – Patients with advance stage ovarian cancer are treated with radical cytoreductive surgery and platinum-based (carboplatin or cisplatin) chemotherapy, and up to 80% of patients are recommended to receive maintenance therapy with poly (ADP-ribose) polymerase (PARP) inhibitors. In a large randomized controlled trial, ovarian cancer patients treated with the PARP inhibitor olaparib had longer progression-free survival than those treated with placebo. However, when patients were re-treated with standard-of-care platinum chemotherapy for presumed platinum-sensitive disease, those who had been treated with olaparib experienced recurrence almost three times faster than those who did not receive a PARP inhibitor (HR 2.89, 95% CI 1.73-4.82)(Frenel et al., 2022, Ann Oncol). This newly identified clinical phenomenon requires immediate attention as it threatens outcomes for most patients diagnosed with ovarian cancer. We are evaluating mechanisms of resistance using model cell lines and patient-derived organoids established in our group and applying a systematic approach, including transcriptomic, immunofluorescence, and functional assays.ReplyReply allForward
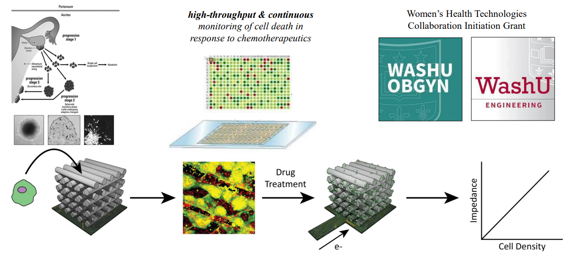
4. In Vitro Bioelectronic Monitoring of Therapy Response in 3D, Patient-Derived Ovarian Cancer Tumor Models: We are working in collaboration with Dr. Alexandra Rutz to investigate the feasibility of using bioelectronic scaffolds to support patient-derived ovarian cancer cells in order to assess the efficacy of various chemotherapeutics and targeted agents against 3D patient tumor models in order to inform patient care in a personalized medicine approach.
- Reproductive Scientist Development Program (RSDP) (Schust, PI) 2021-2023
NIH/NICHD-sponsored national K12 training program, GOG-Foundation
Utilizing DNA Damage to Identify and Target Chemoresistance in Ovarian Cancer
Role: K12 Scholar - Dean’s Scholar Program (Mullen, PI) 2021-2023
Washington University in St. Louis School of Medicine Division of Physician Scientists
Developing ovarian cancer organoid models to establish novel therapies and biomarkers predictive of response to DNA damage therapies
Role: Dean’s Scholar - Collaboration Initiation Grant (CIG) in Women’s Health Technologies 2022-2023
Washington University McKelvey School of Engineering and Obstetrics and Gynecology
In vitro Bioelectronic Monitoring of Therapy Response in 3D, Patient-Derived Ovarian Cancer Models
Role: Co-Principal Investigator