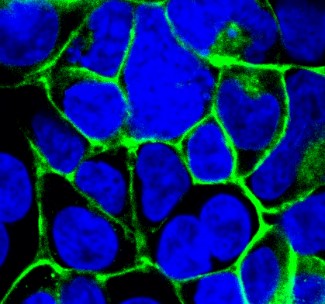
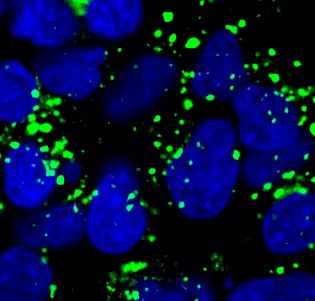
- Mechanisms of oxytocin receptor downregulation
- Identifying positive modulators of the oxytocin receptor
- Role of hypercholesterolemia in myometrial contractility
- Role of S1P-HDL in myometrial contractility
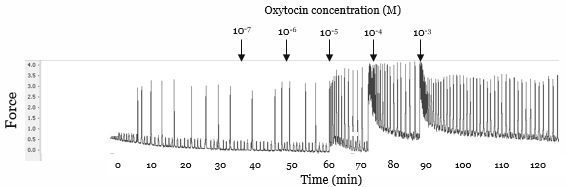
Ex vivo myometrial contractility in response to oxytocin
We utilize mouse models, cells lines, and primary human tissue/cells to study the pathways involved in myometrial contractility.