Prostate cancer
While the focus on understanding factors that contribute to adult malignancy has centered on adult exposures, there is evidence that in utero alterations in energy metabolism resulting in difference in fetal growth contribute to a wide variety of diseases including cancer. Both high and low birth weights in mice and humans have been linked to adulthood cancers such as breast, colorectal, and hematologic malignancies [4, 5].
Recently, the incidence and aggressiveness of prostate cancer in humans have also been associated with increased birthweight. In addition, rodent studies have demonstrated that changes in maternal bioenergetics by dietary alterations shortened latency to developing other endocrine related malignancies such as mammary tumors [9]. Due to the fact that the prostate is hormone responsive and a glandular tissue similar to breast we speculate that prostate cancer may demonstrate the same predisposition based on maternal diet. The mechanism responsible for this phenomenon of in utero exposure to maternal diet resulting in altered energy metabolism and increased disease risk has been studied extensively but no clear-cut answers have been elucidated.
Human and animal studies suggest that in utero environmental exposures result in epigenetic changes that persist following delivery and into adult life. Since substantial evidence supports the role of epigenetic dysregulation in the development of human prostate cancer, we postulate that this dysregulation first occurs as a result of metabolic energy changes in utero, which predispose the prostate to neoplastic transformation.
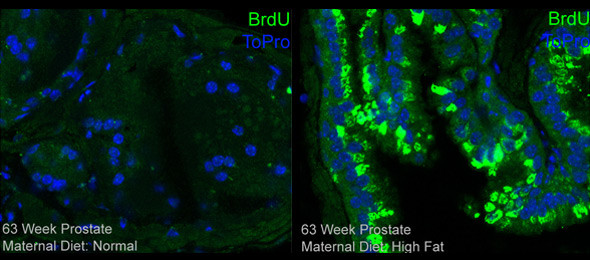
The objective of this project is to investigate the effect of maternal high fat diet and changes in metabolic bioenergetics on prostate development and susceptibility to cancer in male offspring. We hypothesize that a high fat maternal diet prior to and throughout pregnancy alters epigenomic marks leading to abnormal expression of key genes in the prostate gland, which predispose the offspring to develop prostate cancer. Our rationale is that if maternal diet and metabolic bioenergetics alter the process of tumor development and progression in the prostate, then further studies exploring the mechanism of this process may elucidate new therapies and recommendations for the prevention and treatment of prostate cancer.