<<Back to Projects
Oxytocin is used to induce or augment labor in nearly half of women who give birth in the United States. This nonapeptide hormone acts on uterine smooth muscle to initiate, enhance, and pace uterine contractions. However, the effectiveness of a given oxytocin dose varies among women; consequently, oxytocin has a wide therapeutic window. The unpredictability of an individual patient’s sensitivity to exogenous oxytocin and subsequent prolonged oxytocin exposure increases both maternal and fetal risks. Furthermore, our inability to predict a woman’s required dose of oxytocin may lead to a diagnosis of labor dysfunction and thus contribute to the increasing national rate of cesarean deliveries.
Oxytocin induces uterine contractility by binding to the oxytocin receptor (OXTR), a G-protein–coupled receptor. Binding initiates a signaling cascade that results in an increased intracellular calcium concentration and activation of the contractile machinery. To successfully initiate the signaling cascade, OXTR must (1) properly bind oxytocin, (2) interact with its G protein and/or other associated signaling molecules, and (3) translate oxytocin binding to a signal, or conformational change, that activates the G protein. Protein coding variants of OXTR that enhance or interfere with these properties could lead to increased or decreased oxytocin sensitivity. We are seeking to understand the effect of such variants by:
- Identifying genetic variants in the oxytocin receptor that correlate with oxytocin responsiveness
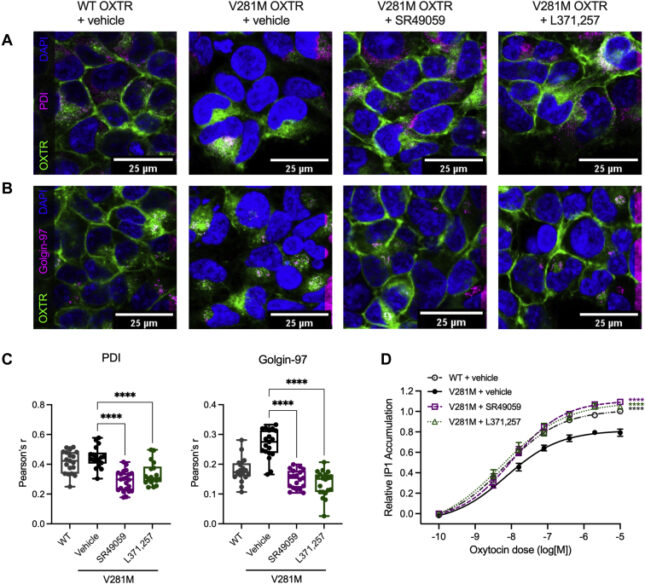
- Examining the use of pharmacoperones to increase surface expression of the oxytocin receptor- link to publication »
- Determining the functional consequence of genetic variants in the oxytocin receptor gene
Published online 2022 Jan 28. doi: 10.1016/j.jbc.2022.101646