Title: Quantification of Neuroinflammation in Alzheimer’s Disease Using Diffusion Basis Spectrum Imaging
Role: Principal Investigator
Our team has previously established the validity of diffusion basis spectrum imaging magnetic resonance imaging (DBSI MRI) for neuroinflammation in multiple sclerosis. We have used this in both in vivo and ex vivo mouse and human studies. We are now able to extend this novel biomarker of neuroinflammation to Alzheimer’s disease (AD).
Our hypothesis is that this relationship will be present in the earliest preclinical stages of AD, when cerebrospinal fluid (CSF) has abnormally low levels of Aβ but before abnormal levels of tau are observed.
We will validate this marker in vivo with positron emission tomography (PET) imaging of regional neuroinflammation. After validation, we will confirm with ex vivo autopsy of human microglial infiltration using immunohistochemistry and autoradiography. DBSI MRI sequences are FDA approved which makes this method ready for clinical trials.

IP Disclosure and Pending
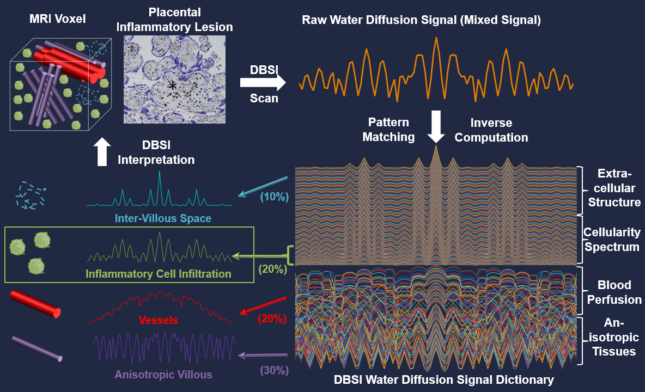
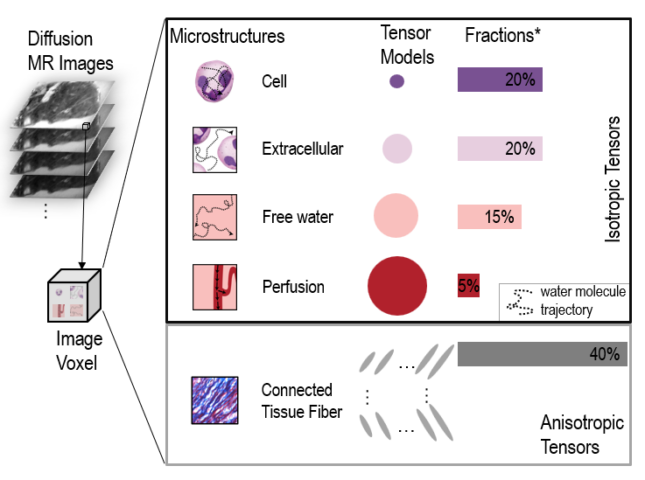
Title: Applying Diffusion Basis Spectrum Imaging to Characterize Human Placenta Immuno-response during the normal term and preterm pregnancies This R01 proposes to develop a novel, noninvasive human placental immune imaging (PII) technique, which will be able to safely assess human placental inflammation in real time. PII will be based on a diffusion magnetic resonance imaging (MRI) technique called diffusion basis spectrum imaging, which can noninvasively image and quantify brain inflammation in multiple sclerosis in both animal models and human patients. Development of PII must take into account the fact that the human placenta is quite different from animal placentas and is a very dynamically changing organ throughout pregnancy. The anatomic and potential pathological complexity and heterogeneity of the placenta create strong background noise interference not present in the brain, making it more challenging to identify and extract the signals specific to placental inflammation. To address this technical challenge, this proposal will develop PII specifically for human applications by making use of three distinct clinical cohorts: those at low risk of preterm birth (Aim 1), and those at high risk of preterm birth who either respond or fail to respond to treatment to prevent preterm birth (Aims 2 and 3). In addition to performing PII on these women at two (Aims 2 and 3) or three (Aim 1) time points in pregnancy, this proposal includes measurement of immune factors and long non-coding RNAs in maternal blood, and histological characterization of inflammatory cells in placenta samples obtained at delivery. Completion of these aims will 1) yield a fully developed PII system, 2) refine PII through histopathological assessments of placentas and measures of systemic immune responses, 3) define the placental immune responses characteristic of healthy, term pregnancy, 4) identify placental immune responses characteristic of pregnancies at risk of preterm birth, and 5) begin to reveal immune signatures associated with success and failure of progesterone treatment.
Reproduction prohibited.
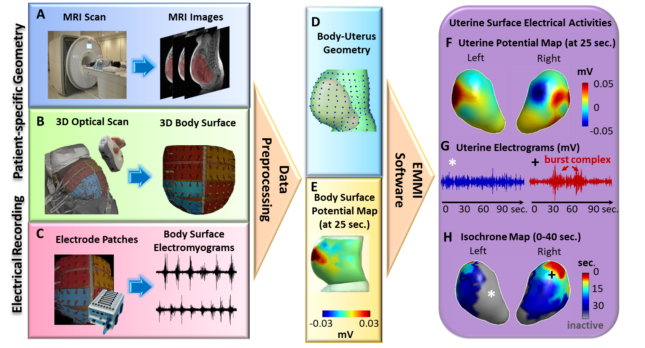
We have limited understanding of how uterine contractions develop and become sufficiently coordinated to expel the fetus at term. For example, we lack answers to basic questions about labor such as where contractions initiate, how fast contractions propagate, which regions of the uterus are active during contractions, and how these measures change as labor progresses. To address this knowledge gap, a new imaging technology, Electromyometrial Imaging (EMMI) was recently developed and validated in a translational sheep model. With EMMI, it is possible to noninvasively image the electrical activation and conduction patterns during contractions across the entire uterus in three dimensions. The objectives of this proposal are to use EMMI to create a “normal term atlas” describing the 3D electrical activation patterns of human uterine contractions at high spatial and temporal resolution across labor, and to use this atlas to begin to identify contraction features associated with impending labor arrest. Aim 1 is to define the uterine electrical maturation and contraction patterns during labor in term nulliparous women. In this Aim, EMMI will be conducted on 430 nulliparous women throughout labor, and data will be analyzed from the 365 women who are anticipated to have normal term labor. This aim tests the hypothesis that at least one of the EMMI-derived uterine contraction indices can precisely reflect progression of normal term labor in nulliparous women, and can reliably differentiate the different phases of the first stage of labor. Exploratory Aim 2 will evaluate uterine electrical contraction patterns during labor in the anticipated 75 women from Aim1 who experience labor arrest. This exploratory aim will provide the basis for a future larger EMMI study to fully characterize the spatial-temporal signatures of uterine contractions in patients who develop arrested labor. In completing these two aims, this project will generate physiologically normal standards of uterine contraction indices of nulliparous women during the progress of term labor. These normal standards will permit future in-depth clinical investigations of the factors leading to dysfunctional labor. Moreover, they may, in the longer term, serve as standards for monitoring pregnancy and labor progression and assessing the effectiveness of treatment strategies to manage labor and prevent labor complications such as preterm birth, labor arrest, and postpartum hemorrhage.
Reproduction prohibited.
Myofascial pain is a significant health concern and financial burden, but no objective and quantitative biomarkers exist for the clinical management of myofascial pain. This project will address this unmet challenge by developing a multi-modal multi-parametric multi-scale approach to identify imaging biomarker(s) that can distinguish different states of myofascial pain and then examining the ability of the identified biomarker(s) to monitor the responses to local chemical injection treatment and predict clinical outcomes in a randomized trial. The proposed research will create vital knowledge about myofascial pain and contribute to the creation of quantitative biomarkers that may guide our choices of appropriate pain management and reduce opioid addiction.
Schizophrenia (SCZ) is a heterogeneous brain disorder typically characterized by delusions, hallucinations, and functional decline, with a typical first onset in late adolescence and early adulthood. Genetic, neuropathological, and neuroimaging studies have suggested a role of neuroinflammation in the etiology of SCZ, which is evident early in the course of illness. This suggests neuroinflammation may represent a SCZ risk marker and therefore facilitate early recognition and future drug development to improve outcomes. In vivo imaging methods for estimating neuroinflammation have been limited by radiation exposure, specificity, and cost. Our proposal aims to validate a novel non-invasive, new magnetic resonance imaging (MRI) technique called Diffusion Basis Spectrum Imaging (DBSI) to identify neuroinflammation in SCZ. DBSI can simultaneously detect and quantify neuroinflammation (increased cellularity) and white matter alterations (axonal injury/loss and demyelination) and has been previously validated in multiple sclerosis and Alzheimer’s disease, but not in SCZ. We propose to test the overarching hypothesis that DBSI will identify neuroinflammation in histological samples from SCZ patients. To achieve this objective, we will obtain postmortem brain samples of 18–30- year-old SCZ patients and matched controls (n=20) from the NIH Neurobiobank and investigate the relationship of the DBSI cellularity subcomponent with tissue reactivity for the microglial marker, CD163, and the complement marker, C4 (Aim 1). We hypothesize a strong linear relationship between DBSI cellularity and selected gray and white matter regions. In addition, we will use DBSI in vivo to characterize the brains of 18–30-year-old SCZ patients and controls (n=30) and identify group differences in DBSI subcomponents (Aim 2). We hypothesize greater DBSI cellularity in SCZ brains compared to controls. In completing this work, we expect to identify non-invasive neuroinflammation and white matter integrity markers for SCZ. In the long term, this information would be used to improve the identification of those at risk for developing psychosis and facilitate the testing of new treatments.
Our short-term goal is to identify interhemispheric mechanisms that support left hand compensation (both performance and use), and determine whether the mechanisms arise from cortical asymmetry for movement (hand dominance). This will provide the foundation for our long-term goal to develop and target therapies to improve compensation for patients who face challenges to rehabilitation due to chronic right hand impairment. Aim 1: identify interhemispheric mechanisms that support left hand performance after right hand injury. Aim 2: identify interhemispheric mechanisms that support left hand usage after right hand injury. Aim 3: determine whether the interhemispheric mechanisms arise from cortical asymmetry.
This project aims to develop and establish a novel compressed sensing-based diffusion MRI technique, diffusion dictionary imaging (DDI), to noninvasively and reliably image neuroinflammation in Alzheimer’s disease (AD). By leveraging the established infrastructure of the Knight Alzheimer’s Disease Research center (P50AG05681, P01AG026276, P01AG003991) and Dominantly Inherited Alzheimer Network (DIAN, U19AG032438), we will cost-effectively develop DDI and use it to study the role of neuroinflammation in the sporadic AD and autosomal-dominant AD. The successful development of a quantitative, endogenous DDI biomarker of neuroinflammation will represent a significant technological leap forward and holds significant promise to improve our understanding of the role of neuroinflammation in AD pathogenesis.
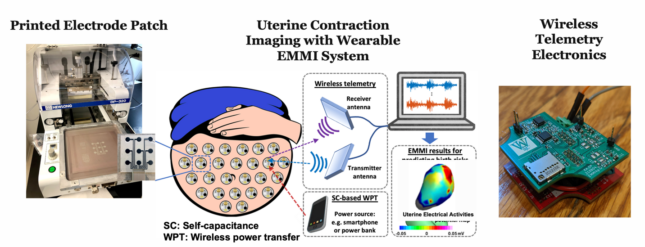
Preterm birth is the greatest and costliest burden in perinatology and it not only results in a high rate of fetal mortality but also puts the children at a lifelong risk of negative health consequences. However, the factors that result in preterm birth are still not very well understood, which is largely due to our limited ability to monitor the human uterine maturation with sufficient spatial and temporal resolutions. We propose to investigate the feasibility of a wirelessly powered, wearable patch system capable of providing high-resolution three- dimensional mapping of uterine surface electrical activity during contractions, which would permit the study of birth-related risks and improve maternal and child outcomes.
Reproduction prohibited.
During pregnancy, the human uterus can expand to 500 times its pre-pregnancy size, remain quiescent for nine months to permit fetal development, produce forceful contractions at term to expel a fetus, and then return to its prepregnancy state. However, we have little understanding of how the uterus accomplishes these feats. For example, whereas we know that heart contractions are coordinated by the sinoatrial node, and intestinal contractions are coordinated by interstitial cells of Cajal, we do not even know whether or not the human uterus has pacemaker cells or early activation regions. Nor do we know how effective uterine contractions differ from ineffective contractions or whether the locations of muscle activation differ from one woman to the next and from one contraction to the next. The project proposed here is to develop a novel patient-specific microstructure-enriched electromyometrial imaging (mEMMI) for uterine contractions. mEMMI will be able to measure electrical activity at the abdomen surface and map it onto the microstructure of the electrically active muscle cells. To develop mEMMI, we will use the team’s expertise in developing two other technologies: diffusion basis spectrum imaging (DBSI), a magnetic resonance imaging technology invented to image brain microstructures, and electromyometrial imaging, a system for mapping electrical activity onto the uterine surface. mEMMI will be developed by pursuing two objectives: 1) Use mEMMI to define the electrical-microstructural features underlying normal labor; and 2) Use mEMMI to describe the uterine microstructural changes and electrical contraction patterns during labor in high-risk pregnant women. Once developed, these studies of the uterus could lead to vast improvements in our ability to solve important pregnancy complications such as preterm birth, which affects ~10% of babies and leads to lifelong negative health consequences. Just as we can now treat fatal heart arrhythmias with substrate-based noninvasive ablation, we envision one-day using mEMMI to pace or modify malfunctioning uterine tissue and thus prevent many cases of preterm birth arising from spontaneous preterm labor. Thus, mEMMI will become a novel imaging tool for next-generation pregnancy research and clinical practice.

Reproduction prohibited.
We invented Electromyometrial Imaging (EMMI) and extensively validated the technology in both a translational sheep model and in humans. EMMI is capable of imaging and quantifying three-dimensional uterine contraction patterns accurately and robustly in a noninvasive fashion. With the essential support from the BMGF, we have developed the critical imaging and electrode components for a portable, low-cost EMMI prototype system. In this project, we will develop an EMMI system that is ready for multi-site clinical trials. We aim to develop and integrate the essential hardware and software components into the clinical trial EMMI system, so it can be easily incorporated and operated independently by clinical researchers and peers at different clinical trial sites. Then, we will validate and quantify UPP in humans using slow-wave EMMI as a novel tool for UPP assessment in human pregnancy. We plan to use EMMI longitudinally over gestation to image UPP in pregnant patients who are at low risk for the development of IUGR and thereby establish the norms for UPP in pregnancy. Once the normal function of UPP is established, we will analyze UPP in pregnancies with a high risk of developing IUGR. The primary goal is to develop and test EMMI-based risk stratification by identifying the pregnancies with increased risk for IUGR.
In this project, we aim to quantitatively and objectively define the myometrial electrical activity underlying uterine peristalsis in each phase of the menstrual cycle. This proposal will contribute to the menstrual health initiative, one goal of which is to establish near-term adjunctive treatments that can address the side effects of heavy and irregular bleeding experienced by some with or without use of hormonal contraceptive methods. Understanding etiologic mechanisms of abnormal uterine bleeding and contraceptive-induced menstrual changes will enable development of products that will be responsive to user needs and preferences and that will mitigate side-effects, leading to increased overall use, continuation, and satisfaction.
In this project, our objective is to fill this knowledge gap by using a method we developed – electromyometrial imaging (EMMI) – to quantitatively and objectively define the myometrial electrical activity underlying UP in three-dimensions. We will use EMMI to test our central hypothesis that measures of myometrial activity during UP (initiation site, direction, duration, and magnitude) will quantitatively differ depending on the phase of the menstrual cycle and will quantitatively differ between women with and without endometriosis.
Maternal opioid use disorder is rapidly increasing in prevalence, and is associated with severe morbidities in offspring, such as neonatal opioid withdrawal syndrome, brain dysmaturation, and impaired neurodevelopment. However, little is known regarding the mechanisms by which maternal OUD causes these outcomes, so our ability to mitigate the impact of prenatal opioid exposure on outcomes is poor. Here, we propose to use longitudinal, advanced diffusion magnetic imaging techniques throughout pregnancy test the central hypothesis that prenatal opioid exposure induces chronic inflammation and hypoxia in the placenta and fetal brain, causing fetal brain dysmaturation, neural injury, and ultimately abnormal ND.
Role: Multiple Principal Investigator for WashU Site
Uterine contractions during pregnancy drive birth, and peristalsis during menstrual cycles not only expels menstrual blood but also plays significant roles in both sperm transport and gynecological disorders. This work developed a 3D patient-specific uterus simulation system called ‘Alya Purple’. Alya Purple provides a virtual platform that integrates clinical data and simulates the propagation of transmembrane potential, while also enabling the simulation of mechanical deformations and fluid dynamics. It highlighted the implications of irregular peristalsis and contractions, particularly in conditions like retrograde menstruation and endometriosis. Furthermore, it explored the therapeutic potential of electrical pacing for various uterine conditions. This tool offer promise for comprehensive diagnostic and therapeutic strategies to enhance the well-being of individuals dealing with pregnancy-related complications and menstrual irregularities.