Maternal-Fetal Immunobiology Lab

Pregnancy showcases a remarkable example of immunological regulation that is meticulously orchestrated by the mother, fetus, and placenta. When this delicate equilibrium is disrupted, it can result in a range of pregnancy complications. Our research is dedicated to investigating the immunobiological pathways that underlie such pregnancy complications, with a special emphasis on preterm birth. We seek to advance our understanding and develop targeted strategies for the prevention and management of preterm birth, ultimately promoting the well-being of both mothers and infants.
Our mission is:
- To conduct translational and basic research on the immunological pathways that lead to obstetrical disorders, with the goals of improving the understanding of the mechanisms of disease and of developing methods for the early diagnosis, treatment, and prevention of pregnancy complications, with an emphasis on preterm birth.
- To train post-graduate and graduate-level professionals in maternal-fetal immunobiology. We welcome MDs and PhDs to be a part of our multidisciplinary group.
Contributions:



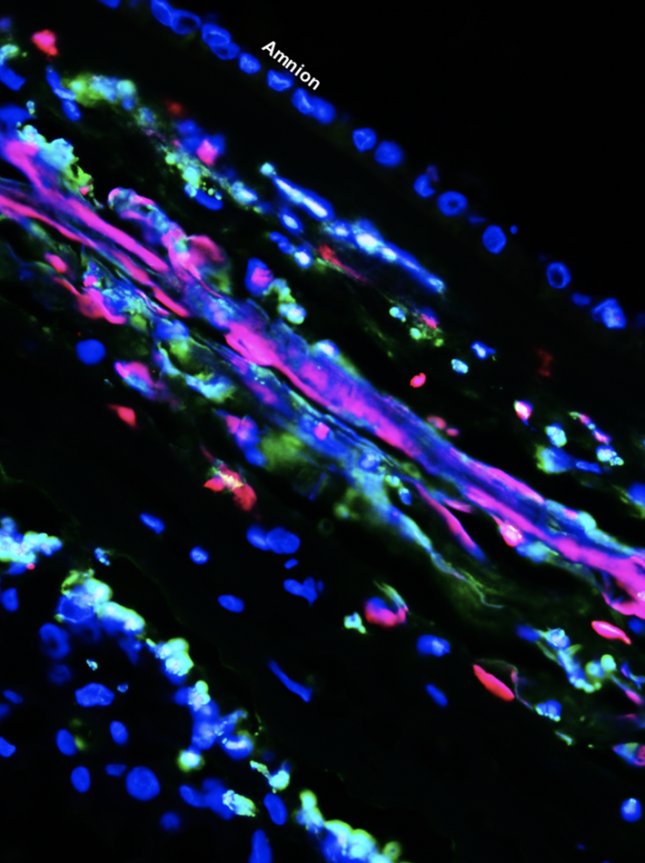
- Maternal T cells reside at the maternal-fetal interface; however, whether such cells were participating in labor was poorly understood. The Gomez-Lopez lab was first to provide evidence for the participation of T cells in the physiologic process of labor. Importantly, we showed that effector and activated T cells are increased at the maternal-fetal interface in a subset of women with idiopathic preterm labor, and that the systemic activation of T cells in mice directly causes preterm delivery [PubMed]. We also demonstrated that the depletion of regulatory T cells from pregnant mice in late gestation similarly results in preterm delivery, and that the restoration of such cells prevents impending preterm delivery, further establishing a causal relationship between aberrant T-cell responses and preterm labor [PubMed]. Notably, we identified a subset of women who undergo idiopathic preterm labor associated with the activation of fetal T cells in the amniotic cavity, a phenomenon that we replicated in mice through the intra-amniotic injection of activated fetal T cells leading to preterm delivery [PubMed].
- Conventionally, maternal macrophages present in the decidua were thought to primarily function as cytokine producers. The Gomez-Lopez lab has helped fill the gap in knowledge regarding the role of macrophages in pregnancy by showing that the decidua hosts a large fraction of macrophages displaying a homeostatic phenotype, regardless of labor, and that women who undergo preterm labor have reduced proportions of homeostatic macrophages [PubMed]. We used transgenic mouse models to demonstrate that the depletion of total macrophages results in preterm delivery, demonstrating a link between homeostatic macrophages and pregnancy maintenance. Based on these findings, we developed a novel cellular approach whereby primary macrophages could be polarized to a homeostatic phenotype in vitro and administered to pregnant mice, thereby preventing inflammation-induced preterm delivery [PubMed].
- The molecular pathways underlying the physiologic and pathologic processes of labor are the center of ongoing investigation. We have previously generated substantial evidence supporting the involvement of inflammasomes, particularly the NLRP3 inflammasome, in women who underwent term or preterm labor. Consistently, animal studies revealed NLRP3 inflammasome activation at the maternal-fetal interface in models of intra-amniotic inflammation. Using transgenic animal models, we have demonstrated roles for the sensor molecule NLRP3 and its inflammasome signaling pathway in the fetal and maternal signaling required for the premature onset of the labor cascade leading to fetal injury and neonatal death, in the context of both microbe-associated [PubMed] and sterile intra-amniotic inflammation [PubMed]. Moreover, such outcomes can be ameliorated by the direct blockade of NLRP3 or by the anti-inflammatory effects of therapeutics approved for use during pregnancy, such as clarithromycin [PubMed].
- Pregnant women represent a high-risk population for severe COVID-19, as evidenced by increased risk of hospitalization, mechanical ventilation, ICU admission, and preterm birth. Early in the pandemic, vertical viral transmission represented a primary area of concern. To address this, we evaluated the human placenta at single-cell resolution and demonstrated a lack of co-expression of the canonical cell entry mediators for SARS-CoV-2, indicating a low likelihood of vertical transmission [PubMed]. However, absence of vertical transmission does not guarantee fetal safety from maternal COVID-19. Therefore, we undertook a comprehensive and multidisciplinary study of pregnant women with COVID-19. We showed that maternal disease can cause fetal inflammation even in asymptomatic patients without evidence of vertical transmission [PubMed], which can improve the care of infected pregnant women to prevent long-term consequences.